In the case of global drug research and development being monopolized by European and American chemical pharmaceuticals for a long time, a pharmaceutical company in Tianjin, China, firmly believes that Chinese medicine is a new driving force for the development of new drugs in the future, and strives to explore a combination of the traditional thinking of traditional Chinese medicine and modern pharmaceutical technology. China's characteristics can also be in line with international standards for the development of new drugs.
"Component Chinese medicine" is a new concept put forward by Yan Xijun, president of the Tianshi Group, at the World Congress of Traditional Medicine held in Beijing on the 8th, and it is also the "breach" of traditional Chinese medicine that this state-level innovative enterprise has explored after six years of practice.
Yan Xijun said that the development of "component Chinese medicine" is based on the original prescription of traditional Chinese medicine, using modern techniques to isolate the effective components of traditional Chinese medicine, clarifying the synergistic effects of various components, ensuring the homogeneity of the active ingredients and establishing pharmaceutical standards. At the same time, the company can also select effective ingredients to form a new drug without any impurities under the guidance of TCM principle of differentiation and treatment, so that its targeted and treatment accuracy is greatly improved.
At present, the nation's first herbal component library has been established in the Tianshili Group. The researchers prepared 10,661 components and 235 compounds from 282 medicinal herbs and 18 proprietary Chinese medicine preparations over a six-year period, and are currently working on the development of four new drugs that are mainly used to treat diabetes, cardiovascular and cerebrovascular diseases, and cranial nerve diseases. And insomnia and so on.
Yan Xijun said that the research and development of "component Chinese medicine" is based on the premise of adherence to the theory of traditional Chinese medicine, based on the resources of traditional Chinese medicine, and in accordance with the relevant laws and regulations of national drug development and examination and approval. It not only maintains the regularities and characteristics of compatibility of traditional Chinese medicine formulas, but also can achieve quality safety, clear composition, clear pharmacology, and also helps to enhance the international recognition of Chinese medicine and promote the internationalization of traditional Chinese medicine.
Bradley Keller, a congenital pediatric cardiologist at the Pittsburgh Children's Hospital in the United States, said in an interview: “Developing new drugs in known Chinese medicine resources is a smart exploration. Although Chinese medicine is mainly based on experience, China People's applications over the centuries constitute the best clinical practice."
Yan Xijun pointed out that the cost of research and development of new medicines through “component Chinese medicine†is likely to double the research and development costs of domestic proprietary Chinese medicines, but this route is still more economical than the development of new drugs in Europe and the United States.
According to statistics from the State Food and Drug Administration, 20 varieties of Chinese herbal medicines currently entered the catalogue of herbal medicines for plants in France, 7 standards for Chinese herbal medicines entered the French Pharmacopoeia, and 4 standards for Chinese herbal medicines entered the European Pharmacopoeia. China has maintained close cooperation in the traditional medicine field with the United States, Canada, France, the European Union, Italy, Australia, Singapore, Thailand and other countries and regions.
Heat Treated Steel Bar And Tube
Heat treated steel bar is produced by heating and cooling in different temperature based on the steel grades to improve the steel bar mechanical properties or machinability for various industrial applications. Heat treated steel bar includes annealed steel bars, Normalized Steel Bar, quenched and temper qt steel bar.
Annealed Steel Bar has better ductility and lower hardness, which can be easier to be machined. The annealing processing is usually widely used for steel grade with higher carbon content above 0.5%. However, some low carbon steel also requires to do annealing for special usage such as 20CrMnTi gear steel. For some special material such as 20CrMnTi gear steel and GCr15 such bearing steel, spheroidizing annealing is often required.
Normalized steel bar sometime is also one kind of annealing processing. It mainly changes the grain to remove the impurities in steel and improves the strength and hardness. For some hot rolled steel bars, to keep the basic mechanical properties, normalizing is often used.
Quenching and Tempering, abbreviated as Q&T is a king of processing that strengthen or harden steel bars by heating the materials and then cooling in water, oil or other liquid medium, that rapidly the change from austenite to perlite to get the proper properties for various usage. The quenched & tempered steel bar materials are usually with carbon from 0.30% - 0.60%, it is widely used as merchant bars in components of various machines.
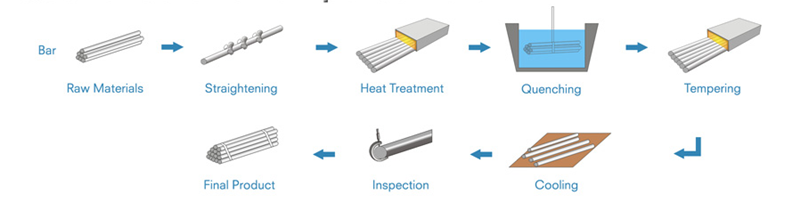
Our advantages on producing heat treated steel bars:
1) Big stocks of hot rolled round bars or wire rods as raw materials
2) Wide range of Cold Drawn Steel Bar sizes: from 10mm to 150mm
3) Different cold drawing medias powder or oil to get different surface
4) Straightening machines to get better straightness up to 0.5mm/m
5) Grinding and polishing machines to get better roughness upto 0.4um
6) Heat treating furnaces to adjust the mechanical properties
7) Full sets of testing equipment to test the sizes, mechanical properties and microstructure.
8) Multiple packages to avoid broken packages and anti-rusty
Heat Treated Steel Bar And Tube
Astm A193,Round Steel Tubing,Heat Treated Steel Tube,Heat Treated Steel Bar And Tube
SHANDONG LE REN SPECIAL STEEL CO., LTD. , https://www.sdthreadedrods.com
